AUSTRALIA has reported its first medical abortion death of a woman who terminated her early pregnancy at home using the abortion pill otherwise known RU-486 or mifepristone.
Little is known about the details and circumstances surrounding her death including; her age, where she died, how many weeks she was pregnant, what type of medical abortion regimen was prescribed to her, or if the medical examiner tested her for a wide range of bacterial infections which includes the lethal Clostridium sordelli pathogen that has resulted in the majority of sepsis deaths in the U.S.
The country’s newspaper, The Australian, reported on March 19, 2012 that a woman died of sepsis “some days after” after she was prescribed an abortion pill regimen at one of Marie Stopes International Australia’s clinics back in 2010. (1) (2)
At that time, the Australian Therapeutic Goods Administration (TGA), Marie Stopes International Australia (MSIA), TGA Medical Officer Dr. Anthony Gill, and well known abortion advocate Professor Caroline De Costa commented on medical abortion issues including; patient care, procedures, and the first known death in Australia from the mifepristone abortion pill.
However, on March 31, journalist Angela Shanahan, for The Australian angrily responded with her opinion about the danger signals in response to the abortion drug death: (47)
Angela Shanahan: “Why did it take two years for her death to become public? RU486 was legalized after great public outcry… Did the authorities not think the medical profession and public might like to know about this?” (47)
Angela Shanahan: "RU-486 (mifepristone) was supposed to be of huge benefit to women. The abortion pill was meant to prevent the problems associated with surgical abortions, the emotional and physical trauma and the ghastly possible complications, and suitable for women in remote areas which, as this poor woman's death shows, it definitely is not." (47)
Obviously, the debate about the risks of medical abortion in Australia is far from over.
This article will also briefly discuss Clostridium sordellii toxic as as an emerging infection following medical abortion, the experimental status of mifepristone in Australia, Marie Stopes International regimens, unapproved regimens used by in the U.S., and the international record of known medical abortion deaths as a result of the prescribed use of mifepristone and misoprostol to terminate early pregnancy.
Medical Abortion Death Confirmed by the TGA
The Australian Therapeutic Goods Administration (TGA) publicly confirmed the death of a woman who was administered the abortion drug in 2010. (2)
The TGA – Australia’s government agency responsible for approving drugs – issued a notice to medical abortion prescribers advising them to take a more active role in following-up with patients who are issued the drug. (1)
It was reported the woman died from group A streptococcus sepsis, a severe bacterial infection of the bloodstream – some days after being prescribed a regimen of two drugs, RU-486 (mifepristone) and misoprostol, from a Marie Stopes International Australia (MSIA) clinic. (1) (2)
Woman Dies under Marie Stopes International Australia (MSIA) Clinical Care
 A review of the MSIA website reveals there is no mention, in its media releases or coverage, of the Australian woman’s death while under their care.
A review of the MSIA website reveals there is no mention, in its media releases or coverage, of the Australian woman’s death while under their care.
Australian media sources reported that MSIA mentioned the coroner's office, in a state it does not name, reviewed the medical reports and other evidence in this case but did not proceed with an inquest and closed the matter. (1) (2)
Both MSIA and the TGA refused to release details of the abortion pill fatality, citing respect for patient confidentiality. (1)
MSIA mentions on their website that medical abortion may be performed from 5 weeks gestation to up to 9 weeks gestation and is an alternative to surgical termination and both have unique advantages and disadvantages. These include the time taken to complete the procedure, the number of clinic visits required, effectiveness, side effects and complications. (3)
- The website doesn’t appear to reveal any information about the risks of medical abortion complications where infection and hemorrhage are the most frequent serious and life-threatening causes of mifepristone related medical abortion illness and death. (4)
- MSIA’s medical abortion FAQ’s doesn’t further disclose that incomplete medical abortion may increase the risk of infection and is associated with discomfort as persistent or recurrent bleeding and pain. (5)
The U.S. Food and Drug Administration (FDA) has already concluded that serious or fatal infection involving the bloodstream after medical abortion may be possibly related to the use of mifepristone and misoprostol. (6)
Australian Medical Abortion Death Case Was Surprising Closed
 Jill Michelson, clinical services director of Marie Stopes International Australia (MSIA) said of the woman's death: "This is a tragic outcome and our sympathies are with her family.”
Jill Michelson, clinical services director of Marie Stopes International Australia (MSIA) said of the woman's death: "This is a tragic outcome and our sympathies are with her family.”
“The coroner dispensed with holding an inquest, and the case is closed." (1)
 Caroline De Costa, a professor of obstetrics and gynaecology at James Cook University in Cairns, says she also accepted that decision. (2) However, she appeared to have some concern about this decision.
Caroline De Costa, a professor of obstetrics and gynaecology at James Cook University in Cairns, says she also accepted that decision. (2) However, she appeared to have some concern about this decision.
“I find it a little surprising but that's, it's… I know nothing more about the case.” (2)
 Angela Shanahan, journalist for The Australian, is not satisfied with these evasive responses as to why the woman's death will not be investigated further.
Angela Shanahan, journalist for The Australian, is not satisfied with these evasive responses as to why the woman's death will not be investigated further.
“The coroner has decided that her death will not be investigated, despite the fact the RU486 was provided in a clinic that is part of the biggest abortion business in Australia, Marie Stopes International Australia.” (47)
Why was this case not important enough to investigate?
An inquest is an official or judicial inquiry that is particularly held to determine the cause and details of a person’s death. (7)
The limited information that has been provided does conclude the woman’s death, confirmed only now, is linked to the use of mifepristone (RU486) since its introduction to Australia in 2006. (1)
Australia’s TGA Medical Officer for Experimental Mifepristone (RU-486)
 In Australia, Dr. Anthony Gill, TGA Medical Officer of the Experimental Products Section, is responsible for mifepristone medical abortion and its communications.
In Australia, Dr. Anthony Gill, TGA Medical Officer of the Experimental Products Section, is responsible for mifepristone medical abortion and its communications.
Monty Patterson of Abortionpillrisks.org website asked Dr. Tony Gill about medical abortion in Australia and details of the 2010 sepsis death of a woman who was reported to have died from Group A Streptococcus (GAS).
Dr. Gill responded briefly with the following points: (8) (9)
- Mifepristone (RU-486) is currently not approved for marketing in Australia.
- Only authorized medical practitioners can prescribe mifepristone as an unapproved therapeutic drug.
- Since mifepristone is not approved for marketing, the TGA does not regulate medical abortion procedures or regimens used in Australia.
- Medical abortion prescribers are responsible for determining the needs of the patient and to monitor the outcome of their own therapy.
- The TGA notice that was previously issued to mifepristone prescribers, after the sepsis death of a woman following medical abortion, was not available for the public’s review.
When asked, Dr. Gill did not provide a response on the following questions:
- Was the patient administered vaginal misoprostol? (All sepsis deaths in the U.S. have resulted with unapproved vaginal use of misoprostol with the exception of one case) (10)
- Did the coroner’s investigation of the patient’s death include specific DNA testing to identify a broad range of bacterial infections that also included the Clostridium sordellii pathogen?
- Clostridium sordellii associated Toxic Shock (CATS) infections following medical abortion have become the leading cause of sepsis death in U.S. women.
- Group A Streptococcus (GAS) infections can cause an illness resembling toxic shock syndrome (Streptococcal Toxic Shock Syndrome or STSS). Persons at higher risk for this invasive disease may include those with compromised immune systems. (11)
- CATS (12) (13) (14)and GAS infections could take advantage of a patient’s immune-compromised condition due to potential anti-glucocorticoid effects of mifepristone that may alter or impair the body’s immune response following medical abortion.
Clostridium sordellii the Emerging Medical Abortion Infection
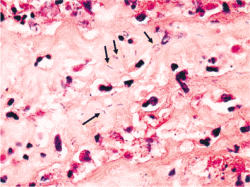 The U.S. Food and Drug Administration (FDA) and U.S. Center for Disease Control (CDC) have acknowledged Clostridium sordellii as an emerging bacterial infection associated with toxic shock following medical abortion with a 100% fatality rate. (15) (16)
The U.S. Food and Drug Administration (FDA) and U.S. Center for Disease Control (CDC) have acknowledged Clostridium sordellii as an emerging bacterial infection associated with toxic shock following medical abortion with a 100% fatality rate. (15) (16)
Clostridium sordellii is present in the vagina in 5% of all women and in up to 29% of women after pregnancy loss. (17)
Symptoms seen with clostridial infections include weakness, nausea, vomiting or diarrhea with or without abdominal pain that persists after expulsion of the pregnancy. Although patients typically lack a fever, they exhibit rapid pulse, low blood pressure, and very high red and white blood cell counts. (18) (19)
Healthcare practitioners in the U.S. have been advised by the FDA to be vigilant so that patients suspected of having an infection, undergoing a medical abortion, are immediately given antibiotics that would treat infections with bacteria such as Clostridium sordellii. (10)
Experimental Medical Abortion in Australia
The Australian Therapeutic Goods Administration (TGA) has allowed the limited introduction of the drug mifepristone to Australia for the purpose of early medical abortion. (20)
TGA authorized prescriber forms on its website state; the agency is unable to vouch for the quality, safety or efficacy of an unapproved product (for example: mifepristone), and that its use is regarded as experimental. (9)
The unapproved prescribed use of the two drug medical abortion regimen of mifepristone and misoprostol for Australian women has been granted special access under an experimental scheme. (21)
Unapproved therapeutic goods have undergone little or no evaluation of quality, safety or efficacy by the Therapeutic Goods Administration. (9) The responsibility for prescribing an unapproved product rests with the prescriber. (9)
This responsibility would apply to all medical abortion prescribers including Marie Stopes International Australia (MSIA).
Australian Abortion Advocate: Professor Caroline De Costa
 Caroline De Costa, a professor of obstetrics and gynaecology at James Cook University in Cairns, has been a prominent advocate for the introduction of medical abortion to Australia. (2)
Caroline De Costa, a professor of obstetrics and gynaecology at James Cook University in Cairns, has been a prominent advocate for the introduction of medical abortion to Australia. (2)
She made headlines when she became the Australian Medical Association’s (AMA) spokesperson on the abortion pill, RU-486. (22)
In 2006, Marie Stopes International Australia (MSIA) congratulated Professor De Costa on her achievement for helping to gain approval to import the first shipment of RU-486 from a New Zealand company. (23)
The revelation that Australia has recorded it first death related to use of the abortion pill RU-486 (mifepristone) has prompted a warning from the Professor De Costa who supported the drug's legalization in Australia. (2)
Professor De Costa Comments About Medical Abortion Deaths
The Recent Medical Abortion Death in Australia
After the woman’s death in Australia, Professor De Costa said her death was caused by an infection that occurred after the abortion. (2)
She stated, “The organism, the bacteria, I have heard was a group A streptococcus." (2)
"This is very susceptible to antibiotic treatment, so it would seem that it's possible if antibiotic treatment had been introduced early for this woman then it might have made some difference, but I really can't say that." (2)
Professor De Costa is worried it is very likely there may be some sort of danger of a backlash against mifepristone (RU-486) because of this death. (2)
She thinks, “We have to be very clear that if we're going to continue using the drug for early medical abortion and using it at home then services have very, very good mechanisms in place for looking after women once they actually leave the clinic.” (2)
De Costa on Medical Abortion Deaths in the United States
In a 2005 medical journal article, Professor De Costa, wrote “It is true that there have been some deaths associated with medical abortions.” (24)
Women have died in the U.S. from sepsis following a mifepristone induced abortion where Clostridium sordellii, a pathogen that produces a powerful lethal toxin, was the causative organism. (24)
The article pointed out that Clostridium sordellii had also been previously reported in American literature as a cause of death after normal or operative vaginal delivery and gynaecological surgery. (24)
Professor De Costa states: “It could be postulated that the women who developed C. sordellii infection after medical termination might equally well have developed the infection had their pregnancies proceeded to term.” (24)
Does Caroline De Costa dismiss the latest Australian 2010 sepsis death of the woman as potentially unavoidable?
De Costa’s statement appears to conclude that a healthy pregnant woman who chooses either a medical abortion procedure or decides to proceed with a full term pregnancy, the choice may be irrelevant because chances are she may be predisposed equally to the risk of fatal infection.
The facts are clear, healthy women have died and have been seriously injured after taking medical abortion drugs to terminate their early pregnancies. (25)
For a healthy woman, medical abortion drugs that lead to a cascade of serious or lethal adverse events are the real problem, not the pregnancy itself.
The risks of severe, life-threatening, or even lethal adverse events, such as infection and sepsis, have been documented in otherwise healthy young women who have used mifepristone and misoprostol for medical abortion. (4) (16)
De Costa did state that she really couldn’t say if antibiotic treatments had been introduced early for the woman then it might have made some difference.
The FDA has concluded that serious or fatal infection involving the bloodstream after medical abortion may be possibly related to the use of mifepristone and misoprostol. (6)
Facts About Infection and Sepsis After Medical Abortion
Sepsis is a potentially life-threatening complication of an infection. (26)
- Sepsis is the most common and dangerous in people who have weakened immune systems. (26)
- Incomplete medical abortion may increase the risk of infection. (5)
- Retention of blood clots and decidual fragments in case of incomplete abortion may increase the likelihood of septic events. (27) (28)
 James A. McGregor M.D., an obstetrician and gynecologist, who has practiced fetal and maternal medicine in Los Angeles, California suggests:
James A. McGregor M.D., an obstetrician and gynecologist, who has practiced fetal and maternal medicine in Los Angeles, California suggests:
"Mifepristone use may impair hosts responses and may predispose to lethal infection caused by toxigenic C. sordellii, other pathogens, and to reexamine the need for mifepristone use in medical termination of pregnancy." (29)
De Costa’s Urgency: The Patient’s Medical Abortion Care
Professor Caroline de Costa is now urging doctors to review the way they care for women who've had a medical abortion. (2)
Some doctors who are authorized to prescribe RU486 (mifepristone) have been told (by the TGA) to review their protocols for caring for patients who have had a medical abortion and that there must be a renewed focus on patient care after the abortion. (2)
"If you are going to have the woman undergoing the abortion processes at home, then you must have very close contact with her.” (2) "And she must know and you must know what arrangements are in place if she should need emergency care or extra care.” (2)
“Infection of retained products of conception (fetal and/or placental tissue) is a possibility, and antibiotics may be needed.” (24)
"There are guidelines from the Royal College of Obstetricians and Gynaecologists (RCOG) in London which recommend using antibiotics prophylactically for all women, or at least considering this when you're practicing early medical abortion. (2)
Angela Shanahan's response to De Costa's statements:
"However, the problem with RU486 is that precisely because it has been encouraged as a method of home abortion, the need for medical follow-up is not a big consideration, especially in the minds of young girls. In the U.S. there have been 14 deaths caused by RU486, and eight of those were because of sepsis. If it is widely marketed by a drug company and, as De Costa and others envisage, replaces most surgical abortions, there may be less involvement of commercial clinics such as MSIA, but a higher rate of complications. The onus for handling these complications will fall on local emergency services." (47)
De Costa Questions Medical Abortion Procedures Used by Providers
Professor De Costa questions the possibility that the protocols for the prescribed use of RU486 in Australia may need to be reviewed. (2)
The procedures used in Australia may vary between prescribers.
De Costa admits once the drug is approved by the TGA to be marketed, she would expect definite medical abortion guidelines to be published either by the TGA or in conjunction with the national licensing of the drug. (2)
Marie Stopes International wouldn't say if it will review the procedures in its 14 Australian clinics. (2)
Marie Stopes International (MSI) says it has administered 18,000 medical abortions in Australia since 2009 using mifepristone (RU486) in combination with a second abortion drug, misoprostol. (2) (1)
At the clinic, the patient receives the standard 200mg tablet of mifepristone to terminate the early pregnancy and is sent home with instructions to take misoprostol 24 hours later to induce uterine contractions to facilitate expulsion of the pregnancy. (1)
Angela Shanahan said the TGA and Marie Stopes International Australia had refused to reveal the protocols for medical abortion to a GP obstetrician who covers emergency services for women who may need treatment for post mifepristone abortions. (47)
Angela Shanahan commented: "In other words, the government's own agency would not reveal the protocols after the use of an abortion drug to an emergency doctor working in a public hospital who might have to pick up the pieces, but instead referred that doctor to the abortion business. A business that is kept afloat by Medicare." (47)
Marie Stopes International Clinics in the U.K. and South Africa
A brief overview of what medical abortion involves at Marie Stopes’ International clinics in United Kingdom states the abortion pill regimen is available before nine weeks of pregnancy which consists of taking two sets of pills (orally) over two visits (this can be on the same day or separate days) which causes the passing of the pregnancy. (30)
In more detail, the Marie Stopes’ clinics in South Africa prescribe a medical abortion pill regimen that can be used for pregnancies up to 9 weeks as follows: (31)
- 1 Tablet (200mg) of Mifepristone will be administered orally at the clinic.
- 4 Tablets (800mcg) of Misoprostol is taken at home within 24-48 hours of taking the Mifepristone at the clinic; 2 under the tongue and 1 tablet in each cheek.
- The process can take 14 days to complete. Pain and heavy bleeding should subside once the pregnancy has been expelled. Lighter bleeding will continue for 2-3 weeks.
- A follow up visit to the clinic must be undertaken 3 weeks after taking the misoprostol medication.
- If the abortion is not complete misoprostol may be administered as a repeat dosage with another follow up visit 24 hours later.
Unapproved Medical Abortion Regimens Used in the U.S.
 In 2000, the US Food and Drug Administration (FDA) approved a mifepristone/misoprostol medical abortion regimen protocol for termination of pregnancies up to 49 days (7weeks) from the first day of the woman’s last menstrual period (LMP). (16)
In 2000, the US Food and Drug Administration (FDA) approved a mifepristone/misoprostol medical abortion regimen protocol for termination of pregnancies up to 49 days (7weeks) from the first day of the woman’s last menstrual period (LMP). (16)
Once medical abortion providers in the U.S. had FDA approval for mifepristone and misoprostol pregnancy termination, abortion researchers set out to increase the gestational age limit, shorten the process, decrease their costs, change the dosing regimen, allow women to administer misoprostol at home (instead of at the provider's clinic) and reduce the number of patient’s return visits back to the clinic. (32)
The FDA-approved regimen, an oral combination of 600 mg mifepristone followed 2 days later by office-based administration of 400 mcg of misoprostol, has been used in only 4% (4 per 100) of abortion provider facilities since its approval in 2000. (33)
The FDA has issued public health advisories to warn health care professionals about the risks of sepsis in medical abortion and to reinforce use of the approved regimen which does not include the vaginal use of misoprostol. (34) (35)
International Medical Abortion Deaths – All Causes
Since 2001, at least twenty-one women worldwide have died from fatal complications including hemorrhage, toxic shock, sepsis, organ failure, and ruptured ectopic pregnancy following early medical abortion with mifepristone and misoprostol. (1) (2) (36)
There have been media reports of a European manufacturer's mention of the potential for additional deaths due to mifepristone and misoprostol medical abortion. It appears there is an official lack of documentation at this time.
International Medical abortion Deaths – Sepsis/Toxic Shock
Bacterial Infections and sepsis (a serious infection involving the bloodstream)
Since 2001, eleven women—one in Canada, eight in the United States, one in Portugal, and one in Australia—have died from sepsis/toxic shock syndrome (nine from Clostridium sordellii, one from Clostridium perfringens, one from Group A Streptococcus) following early medical abortions with mifepristone and misoprostol. (1) (10) (16) (37) (38) (39)
- 9 women were diagnosed with Clostridium sordellii fatal toxic shock syndrome,
- 1 woman was diagnosed with Clostridium perfringes,
- 1 woman is believed to have died from Group A Streptococcus (GAS)
Medical Abortion Poor Monitoring and Unreliable Reporting
Due to poor monitoring, unreliable and voluntary reporting to international authorities, it is unknown how many women may have died from infections following medical abortion. (40)
A medical abortion death may only become public knowledge if a report is submitted to a governmental agency or if a person or patient goes to the media to publicize the incident.
Conclusion
 Medical abortion with mifepristone and misoprostol has been an expanding alternative to surgical abortion. It has been accelerating in line with experience in the U.S. and Europe, where up to half of all of elective terminations in Australia are now carried out with mifepristone. (1)
Medical abortion with mifepristone and misoprostol has been an expanding alternative to surgical abortion. It has been accelerating in line with experience in the U.S. and Europe, where up to half of all of elective terminations in Australia are now carried out with mifepristone. (1)
The risks of medical abortion can have a serious impact on a woman’s health. Taking an abortion pill may seem to be very easy, but can result in infection and complications that are serious and life-threatening which could lead to hospitalization or death. (18)
Women worldwide deserve to know all the facts and to be well-informed of all potential risks to their health and welfare when considering an early medical abortion termination with mifepristone and misoprostol. (41)
Professor De Costa even stated in her own book, RU-486, that it needs to be made clear that: (42)
“Medical abortion using mifepristone and misoprostol is not a Magic Bullet that simply melts the pregnancy away.”
REFERENCES
1. Walker, Jamie. National Affairs, Abortion pill death sparked warning. The Australian. [Online] March 19, 2012. [Cited: March 19, 2012.] http://www.theaustralian.com.au/national-affairs/health/abortion-pill-death-sparked-warning/story-fn59nokw-1226303297539.
2. Donovan, Samantha, The World Today, ABC: The Australian Broadcasting Corporation. Death may spark backlash against abortion drug. ABC News. [Online] March 19, 2012. [Cited: March 19, 2012.] http://www.abc.net.au/news/2012-03-19/ru486-death-prompts-protocol-review/3899084.
3. Marie Stopes International Australia. Medical Abortion, dr marie services. Marie Stopes International Australia. [Online] 2012. [Cited: March 28, 2012.] http://www.mariestopes.org.au/our-services/women/abortion/medical-abortion.
4. Gary, Margaret M and Harrison, Donna J. Analysis of Severe Adverse Events Related to the Use of Mifepristone as an Abortifacient, Annals of Pharmacotherapy, 40(2): 191-7. NCBI, PubMed. [Online] February 2006. [Cited: September 13, 2011.] http://www.ncbi.nlm.nih.gov/pubmed/16380436. PMID: 16380436.
5. Rorbye, C, Norgaard, M and Nilas, L. Prediction of late failure after medical abortion from serial β‐hCG measurements and ultrasonography, European Society of Human Reproduction and Embryology, Volume 19, Issue1, Pp. 85-89. Oxford Journals, Human Reproduction. [Online] 2004. [Cited: September 8, 2011.] http://humrep.oxfordjournals.org/content/19/1/85.full. Online ISSN 1460-2350 – Print ISSN 0268-1161.
6. U.S. Department of Health & Human Services. Drugs, Mifeprex Questions and Answers, 2/24/2010. FDA, U.S. Food and Drug Administration. [Online] February 24, 2010. [Cited: July 16, 2011.] http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm111328.htm.
7. Merriam Webster. Inquest. Merriam Webster Dictionary. [Online] 2012. [Cited: March 27, 2012.] http://www.merriam-webster.com/dictionary/inquest.
8. Gill, Anthony. Personal Phone Call Communications: Australian Medical Abortion Death Reported in the Media. Livermore, California : Monty L Patterson of AbortionPillRisks.org, March 20, 2012.
9. Australian Government, Therapeutic Goods Administration. Health Professionals, Accessing Unapproved Products. Department of Health and Ageing, Therapeutic Goods Administration (TGA). [Online] March 26, 2012. [Cited: March 26, 2012.] http://www.tga.gov.au/hp/access.htm.
10. U.S Department of Human & Health Services, U.S. Food and Drug Administration. Mifepristone Questions and Answers, 4/17/2002. U.S Department of Human & Health Services, U.S. Food and Drug Administration. [Online] April 17, 2002. [Cited: June 29, 2011.] http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm111354.htm#6.
11. CDC, Center for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases: Division of Bacterial Diseases. Group A Streptococcal (GAS) Disease. Department of Health and Human Services, CDC. [Online] April 3, 2008. [Cited: March 27, 2012.] http://www.cdc.gov/ncidod/dbmd/diseaseinfo/groupastreptococcal_g.htm.
12. Miech, Ralph P. Disruption of the innate immune system by mifepristone and lethal toxin of Clostridium sordellii. Journal of Organ Dysfunction. 2007, pp. 1-5, i-First article.
13. Miech, Ralph P. Pathophysiology of Mifepristone-Induced Septic Shock Due to Clostridium sordellii, Annals of Pharmacotherapy, Vol 39(9). The Annals of Pharmacotherapy. [Online] September 2005. [Cited: September 12, 2011.] http://www.theannals.com/content/39/9/1483.abstract. doi: 10.1345/aph.1G189.
14. Department of Health & Human Services, CDC, FDA, NIH. Emerging Clostridial Disease Workshop, May 11, 2006, James McGregor, pages 15-17. U.S. Department of Health & Human Services, U.S. Food and Drug Adminstration. [Online] June 22, 2006. [Cited: June 28, 2011.] http://www.fda.gov/downloads/AboutFDA/CentersOffices/CDER/UCM183030.pdf.
15. Aldape, M J, Bryant, A E and Stevens, D L. Clostridium sordellii Infection: Epidemiology, Clinical Findings, and Current Perspectives on Diagnosis and Treatment, Clinical Infectious Diseases, 43(11): 1436-1446. NCBI, PubMed. [Online] December 1, 2006. [Cited: September 13, 2011.] http://www.ncbi.nlm.nih.gov/pubmed/17083018. PMID:17083018.
16. U.S. Department of Health & Human Services. Drugs, Mifeprex (mifepristone) Information. FDA, U.S. Food and Drug Administration. [Online] July 19, 2011. [Cited: July 19, 2011.] http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm111323.htm.
17. Worchester, Sharon. Mifepristone Deaths Raise Unanswered Questions, James A. McGregor, M.D. Ob.Gyn.News, Volume 40, Issue 19, page 13. [Online] October 2005. [Cited: June 28, 2011.] http://www.obgynnews.com/article/S0029-7437(05)71053-3/preview.
18. U.S. Department of Health & Human Services. Drugs, Labeling and Regulatory History from Drugs@FDA, Mifeprex (mifepristone) Medication Guide, Rev 3: 4/22/09. FDA, U.S. Food and Drug Administration. [Online] July 19, 2011. [Cited: July 20, 2011.] http://www.fda.gov/downloads/Drugs/DrugSafety/UCM088643.pdf.
19. National Abortion Federation. A Provider's Guide to Medical Abortion, Complications of Medical Abortion. National Abortion Federation, Early Options,. [Online] 2010. [Cited: August 2, 2011.] http://www.prochoice.org/education/cme/online_cme/m2complications.asp.
20. De Costa, CM, et al., et al. Introducing early medical abortion in Australia: there is a need to update abortion laws. NCBI, PubMed, US National Library of Medicine, National Institutesd of Health. [Online] December 4, 2007. [Cited: March 27, 2012.] http://www.ncbi.nlm.nih.gov/pubmed/18082063.
21. Australian Government, . Special access scheme. Department of Health and Ageing, Therapeutic Goods Administration (TGA). [Online] March 27, 2012. [Cited: March 27, 2012.] http://www.tga.gov.au/hp/access-sas.htm.
22. Australian Broadcasting Corporation. ABC Local, Conversations with Richard Fidler, Caroline de Costa and Sam Rainsy. ABC, Australian Broadcasting Corporation. [Online] February 22, 2007. [Cited: March 26, 2012.] http://www.abc.net.au/local/stories/2007/02/22/1854383.htm.
23. Marie Stopes International. Medical abortion using methotrexate – pilot program. Dr Marie, Abortion Help. [Online] April 07, 2006. [Cited: March 26, 2012.] http://www.abortionhelp.com.au/news/media-releases/79-medical-abortion-using-methotrexate–pilot-program–abortion-help.
24. De Costa, Caroline M. Medical abortion for Australian women: it's time, MJA, Volume 183, Number 7. The Medical Journal of Australia. [Online] October 3, 2005. [Cited: March 24, 2012.] https://www.mja.com.au/journal/2005/183/7/medical-abortion-australian-women-its-time#8.
25. Patterson, Monty L. Abortion Pill Risks, Just the Facts, Risk Warnings: Medical Abortion Adverse Reactions. Abortion Pill Risks. [Online] August 29, 2011. [Cited: March 28, 2012.] http://abortionpillrisks.org/risk-warnings/adverse-reactions/#postmarket-adverse-events-us.
26. National Institute of Health. Sepsis Fact Sheet. National Institute of General Medical Sciences. [Online] January 30, 2012. [Cited: March 27, 2012.] http://www.nigms.nih.gov/Education/factsheet_sepsis.htm.
27. Sitruk-Ware, Regine. Mifepristone and misoprostol sequential regimen side effects, complications and safety, Contraception 74 (1), 48-55. NCBI, PubMed. [Online] March 20, 2006. [Cited: September 12, 2011.] http://www.ncbi.nlm.nih.gov/pubmed/16781261. PMID: 16781261.
28. Sitruk-Ware, Regine and Spitz, Irving M. Pharmacological properties of mifepristone: toxicology and safety in animal and human studies, Contraception, 68(6): 409-20. NCBI, PubMed. [Online] June 17, 2003. [Cited: September 12, 2011.] http://www.ncbi.nlm.nih.gov/pubmed/14698070. PMID: 14698070 .
29. McGregor, James A and Equiles, Ozlem. Risks of mifepristone abortion in context, Contraception, 72(5): 393; author reply 394. NCBI, PubMed. [Online] November 2005. [Cited: September 12, 2011.] http://www.ncbi.nlm.nih.gov/pubmed/16246668. PMID: 16246668.
30. Marie Stopes International. Medical abortion (the abortion pill). Marie Stopes International. [Online] March 26, 2012. [Cited: march 26, 2012.] http://www.mariestopes.org.uk/Womens_services/Abortion/Abortion_options/Medical_abortion.aspx.
31. Marie Stopes South Africa. Medical Abortions, The Marie Stopes Medical (Abortion) Process. Marie Stopes South Africa. [Online] March 26, 2012. [Cited: March 26, 2012.] http://www.mariestopes.org.za/medical_abortions.aspx.
32. Schaff, Eric A. Mifepristone: ten years later . Contraception, Volume 81, Issue 1 . October 2010, pp. 225-229.
33. Wiegerinck, Melanie MJ, et al., et al. Medical abortion practices: a survey of National Abortion Federation members in the United States, Contraception, Vol.78, Issue 6. pages 492-499. NCBI, PubMed. [Online] December 2008. [Cited: September 14, 2011.] http://www.ncbi.nlm.nih.gov/pubmed/19014795. PMID: 19014795.
34. U.S Department of Health & Human Services. Drugs, Public Health Advisory: Sepsis and Medical Abortion, November 4, 2005 Update. FDA, U.S. Food and Drug Administration. [Online] November 04, 2005. [Cited: July 16, 2011.] http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm051734.htm.
35. U.S. Department of Health & Human Services. Drugs, Public Health Advisory: Sepsis and medical abortion with mifepristone (Mifeprex). FDA, U.S. Food and Drug Administration. [Online] March 17, 2006. [Cited: July 13, 2011.] http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm051298.htm.
36. U.S. Department of Health & Human Services. Drugs, Postmarket Drug Safety Information for Patients and Providers, Related Information, Mifeprex Adverse Events Report as of April 2011. FDA, U.S. Food and Drug Administration. [Online] April 30, 2011. [Cited: July 19, 2011.] http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM263353.pdf.
37. Reis, T, et al., et al. A Clostridium sordellii fatal toxic shock syndrome post-medical-abortion in Portugal, Abstract number: R2542. ESCMID, European Society of Clinincal Microbiolgy and Infectious Diseases, Milan, Italy. [Online] May 7-10, 2011. [Cited: September 12, 2011.] http://www.eccmidabstracts.com/abstract.asp?id=93762.
38. Patterson, Monty L. Health Risks: Medical Abortion Deaths. Abortion Pill Risks, Just the Facts. [Online] 2012. [Cited: March 28, 2012.] http://abortionpillrisks.org/health-risks/deaths/.
39. Sinave, Christian, et al., et al. Toxic Shock Syndrome Due to Clostridium sordellii: A Dramatic Postpartum and Postabortion Disease, Volume 35, Issue 11, pp. 1441-1443. Oxford Journals, Clinical Infectious Diseases. [Online] August 7, 2002. [Cited: September 12, 2011.] http://cid.oxfordjournals.org/content/35/11/1441.full?sid=a2610f54-ca62-403f-bce5-78e2eabb4bcf. doi: 10.1086/344464.
40. Creinin M, etal. Mifepristone-Misoprostol Medical Abortion Mortality. Medscape General Medicine. [Online] April 14, 2006. [Cited: September 2, 2010.] http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1785176/.
41. Patterson, Monty L. Abortion Pill Risks, Just The Facts. Abortion Pill Risks. [Online] 2012. [Cited: March 28, 2012.] http://abortionpillrisks.org/.
42. Costa, Caroline De. RU-486, The Abortion Pill. Salisbury : Boolarong Press, 2007. 9781921054334.
43. National Abortion Federation. Module 1, Pharmacolgical Approaches to Early Abortion. Early Options, National Abortion Federation. [Online] 2010. [Cited: June 29, 2011.] http://www.prochoice.org/education/cme/online_cme/m1pharma.asp.
44. Jones, Rachel and Henshaw, Stanley. Mifepristone for Early Medical Abortion: Experiences in France, Great Britain and Sweden. Guttmacher Institute. [Online] May/June, Volume 34, Number 3 2002. [Cited: January 18, 2011.] http://www.guttmacher.org/pubs/journals/3415402.html.
45. Walker, Jamie. National Affairs, Abortion Pill 'less safe than surgery'. The Australian. [Online] May 07, 2011. [Cited: March 20, 2012.] http://www.theaustralian.com.au/national-affairs/abortion-pill-less-safe-than-surgery/story-fn59niix-1226051434394.
46. U.S. Department of Health & Human Services. Label, Mifeprex (mifepristone) Tablets, 200mg, Rev 4:4/22/09. Drugs @FDA, U.S. Food and Drug Administration. [Online] April 27, 2009. [Cited: July 20, 2011.] http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020687s015lbl.pdf.
47. Shanahan, Angela. Danger signals in TGA response to abortion drug death. The Australian, National Affairs, Opinion. [Online] March 31, 2012. [Cited: March 31, 2012.] http://www.theaustralian.com.au/national-affairs/opinion/danger-signals-in-tga-response-to-abortion-drug-death/story-e6frgd0x-1226314778395.



{ 1 comment… read it below or add one }
Very well-researched article