First Global Medical Abortion Death from Fatal Clostridium Septicum Infection
 United Kingdom: A 31-year-old U.K. woman has died of an aggressive and rare infection after using mifepristone (Abortion Pill, RU-486) and misoprostol as reproductive healthcare providers and researchers continue to unravel the association of fatal infections linked to medical abortions. (1)
United Kingdom: A 31-year-old U.K. woman has died of an aggressive and rare infection after using mifepristone (Abortion Pill, RU-486) and misoprostol as reproductive healthcare providers and researchers continue to unravel the association of fatal infections linked to medical abortions. (1)
A case report published in the July 2013 issue of Journal of Obstetrics and Gynaecology confirms the discovery of the first known global death case of “Fatal Clostridium septicum following medical termination of pregnancy.” (1)
Clostridium septicum infection in pregnancy is rare. Infections of the uterus with clostridial anaerobic bacteria are relatively uncommon, but they are among the most fatal. (2)
Since 2001, including the U.K. death, twelve women worldwide have been documented to have died from rare fatal bacterial infections involving sepsis, toxic shock syndrome, and gas gangrene after medical abortion with mifepristone and misoprostol. (3)
Cheen Leen Khoo M.D. and her colleagues clinically detailed the rapid and fatal complications experienced by the U.K. patient while in the British hospital in 2010. (1) Outside of the case report findings, Dr. Khoo provided additional information concerning the woman’s prior medical abortion history to give greater insight into this important case. (4) (5)
Note: This United Kingdom 2013 case report has been reported to the Centers for Disease Control and Prevention (CDC) and the United States Food & Drug Administration (FDA). (6) (7)
MedWatch can be used to voluntarily report a serious problem, product use error, or adverse event associated with the use of an FDA-regulated drug.
The U.K. Patient’s Treatment Regimen
A month prior to the U.K. woman being admitted to the hospital, she had been prescribed a medical abortion pill regimen of 200 mg mifepristone orally (at the hospital) and discharged to complete the rest of the procedure at home. Two days later, the patient vaginally inserted 800 mcg misoprostol and four hours later repeated with a dose of 400 mcg misoprostol. (5)
The registered European medical abortion protocol requires the woman to take 600 mg of oral mifepristone in a licensed facility, return two days later to the clinic and take 400 mcg oral misoprostol, and return 14 days later after mifepristone for a follow-up visit. (8)
Off-label variants of registered regimens include a reduced dose of 200 mg oral mifepristone followed by 800 mcg vaginal misoprostol, which has become common in clinical practice in the United Kingdom. Although this regimen has been recommended by the Royal College of Obstetricians and Gynaecologists and the World Health Organization, it has never been approved by any regulatory agency. (8) (9)
A number of different off-label or alternative medical abortion regimens have been recommended by providers in the United States. In the United States the FDA has issued public health advisories to warn health care professionals about the risks of sepsis and toxic shock in medical abortion and to reinforce use of the FDA approved regimen which does not include the vaginal use of misoprostol. (10) (11)
Prophylactic Antibiotic & The Follow Up Appointment
The U.K. patient was prescribed a prophylactic antibiotic, Doxycycline, to take for 10 days on her discharge home. (4) This antibiotic was given to a patient to take as preventative measure against infection.
Additionally, a follow-up appointment was scheduled for the patient to return a week later, after initiation of medical abortion, to evaluate the degree of bleeding, check for any signs of infection, and confirm the pregnancy is completely terminated. (12) (13)
According to Dr. Khoo, the patient failed to attend her follow-up evaluation. The next time she returned to the hospital was for her fatal admission. (4)
Hospital Admission & Her Fatal Complications
Please read the case report for the exact clinical symptoms, treatment, diagnosis, and discussion. (1)
In 2010, a 31 year-old woman was admitted to the hospital at the Royal Albert Edward Infirmary in the United Kingdom. The woman had five pregnancies in her life, including the last termination; three pregnancies beyond 20 weeks and two pregnancies less than 20 weeks. (5)
The patient had experienced a one day episode of abdominal pain and vaginal bleeding, commencing one month after a medical pregnancy termination at 8 weeks gestation. She was given a transvaginal pelvic ultrasound scan. The results did not reveal any placental and/or fetal tissue remaining in the uterus following medical abortion.
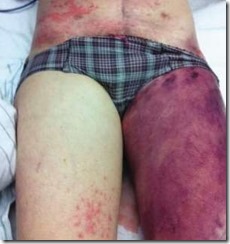
Approximately 22 hours after the patient was admitted to the hospital she started to complain of cramping in her legs. A cascade of septic events followed; bruising, swelling, intramuscular bleeding on her thigh and buttock had increased significantly in an inflammatory response to tissue damage and excessive blood clotting that had formed throughout small blood vessels. The patient had become drowsy, confused and septic. Her condition continued to rapidly deteriorate.
A surgical procedure was performed, involving a large incision through the abdominal wall to gain access into the abdominal cavity, to look for the cause of sepsis that could have triggered these serious adverse events. No source of intra-abdominal or pelvic sepsis was found.
Swelling and the escape of blood into the tissues from ruptured blood vessels around her thighs and gluteal region significantly increased.
Blisters containing blood had appeared on her thighs, gas gangrene along with necrotic foul-smelling tissue extending to the bone was noted upon incising the thigh skin.
In spite of life saving measures being carried out, the patient suffered a cardiac arrest. She died 28 hours after being admitted to the hospital and only 5.5 hours following the onset of leg pain.
What the Autopsy Revealed
Postmortem examination revealed inflammation and abscess formation in the uterus at the site of the patient’s pregnancy termination. There was necrosis (death) of the skeletal muscle predominately in the thighs, with infiltration by Gram-positive organisms and no inflammatory cell infiltration. Blood cultures grew anaerobic Clostridium septicum. (1)
The cause of death was thought to be Clostridium septicum septicaemia secondary to medical termination of pregnancy. (1)
What May Have Caused the Fatal Infection?
The case report of the 31-year-old United Kingdom woman suggested; vaginal carriage of Clostridium septicum is viewed to be the most likely source of infection with the inflammatory focus in the uterus from the site of the terminated pregnancy thought to be the portal of entry for this organism, causing the fatal outcome. (1)
Vaginal bleeding and dilation of the cervix promoted by misoprostol during a medically induced abortion can allow the passage of vaginal pathogens or bacteria to ascend into the uterus that can potentially lead to an infection of the endometrium. This critical event can result in the onset of sepsis, septic shock and death. (14) (15)
See the medical abortion video animation at the Abortion Pill Risks website to learn how a bacterial infection can ascend into the uterus.
Researchers and medical doctors have also suggested that mifepristone and misoprostol each have their own specific mechanisms of drug action that may enhance or predispose women to the development of lethal infection by interrupting, suppressing or altering innate immune responses. (14) (15) (16) (17) (18)
Clostridium Septicum Facts
Severe infection with Clostridium septicum in healthy humans is relatively rare. The organism has been a well-known complication of war wounds in the form of gas gangrene. C. septicum produces exotoxin which is responsible for rapid progression of infection. (19)
The term "gas gangrene" to those invasive anaerobic infections of muscle are characterized by profound toxemia, extensive local oedema, massive death of tissue, and a variable degree of gas production. (2)
Clostridium septicum is a rare life-threatening infection that usually affects patients with some degree of trauma, malignant disease, vascular insufficiency or drug-induced immune suppression. Infection with this organism is increasingly found to be a cause of septicemia, toxic shock syndrome and most commonly associated with spontaneous or non-traumatic gas gangrene. (20) (21) (22) (23) (24)
 The trauma need not be very severe. Provided the wound is deep and necrotic and shut off from the surface, it is always possible for an anaerobic infection to become established. (2)
The trauma need not be very severe. Provided the wound is deep and necrotic and shut off from the surface, it is always possible for an anaerobic infection to become established. (2)
It is also worth noting that clostridial spores may remain dormant in scar tissue for considerable periods and then give rise to gas gangrene years after the original injury as a result of quite minor degrees of trauma, which need not even involve an open lesion. (2)
The infecting organisms may be found as mere contaminants, as saprophytes subsisting in endometrial and placental debris, even within a dead fetus or as true invaders of healthy, intact, uterine muscle. (2)
Spontaneous gas gangrene caused by Clostridium septicum is a rapidly progressive tissue infection with prominent morbidity and mortality. Mortality rates ranges from 67 to 100%, with the majority of deaths occurring with 24 hours of onset. (20)
Early identification and emergent action is necessary for proper treatment. Timely diagnosis of gas gangrene is of the utmost importance. It often can be overlooked as a benign soft-tissue infection in the early stage. No specific diagnostic criteria exist; however, a triad of pain out of proportion to the injury, tachycardia unexplained by fever, and crepitus in the soft tissue is strongly suggestive. A history of recent open trauma, surgery, diabetes, or an immune-compromised state is important to elicit. (22)
Other Cases of Clostridium Septicum in Pregnancy
A single case of Fatal Clostridium septicum infection in a 27-year-old pregnant woman who had presented to a hospital with a spontaneous abortion at nine weeks’ gestation has been previously discussed in the 2006 Netherlands Journal of Medicine. She died within 30 hours after admission to the hospital’s intensive care unit. (20)
The only known case of survival from Clostridium septicum infection in a young pregnant patient was reported in a BMJ 2012 case report. The patient had presented to a rural emergency department with vaginal bleeding at 7 weeks of gestation. She required aggressive resuscitation and surgical management of a septic abortion. The patient's condition improved rapidly following surgical evacuation of the uterus with dilatation and curettage. (25)
A Doctor’s Message To Women Considering Medical Abortion
Dr. Khoo said, “I think it is important for women to understand what the risks are associated with any procedure they undergo; and that the relevant statistics are given to these women; including alternative options & risks of those (such as carrying a pregnancy to term – mortality risks with that is higher).”
“I think in this day and age, very few women actually expect to die when they decide to have a baby; and fewer still when they decide for a termination of pregnancy.” (5)
Worldwide Medical Abortion Deaths from Infection: Limited Surveillance & Reporting
The reported cases of death due to medical pregnancy termination are considered rare, about 1:100,000 in the first trimester. The reported risk of death carrying a pregnancy to full term is approximately 1:10,000. (9)
Worldwide, the true incidence of lethal pathogens following medical abortion is largely unknown due to unreliable surveillance and reporting. The meticulousness of monitoring and the reliability of reporting from much of the international medical abortion community, especially from China, are not well delineated. (9)
In the United States, reporting of abortion procedures and complications is a passive and voluntary process. Also, women may not report having had an abortion when presenting to a hospital with complications therefore the true incidence of serious adverse events and death may be unknown. (26)
CDC reporting systems usually do not indicate specific causative organisms for deaths from infection, and information that would confirm causation is not available. Identifying rare pathogens may be a minimum number and the frequency of fatal infection in pregnancy remains unknown. (27)
The U.S Food and Drug Administration (FDA) relies on the voluntary reporting of serious adverse events by consumers and healthcare professionals through its MedWatch reporting program. Commonly, the FDA states that between 1 to 10 percent of all adverse drug events are reported. (28) (29)
Global Cases of Fatal Infections After Medical Abortion
Since 2001, twelve women in North America, Europe and Australia who had medical abortions with mifepristone and misoprostol have died from bacterial infections caused by sepsis, toxic shock syndrome and gas gangrene. Nine deaths were associated with the lethal Clostridium sordelli infection, one from Clostridium perfringens, one from Group A Streptococcus, and one from Clostridium septicum. (3)
The U.S. FDA and the CDC have documented a total of twelve rare infectious deaths after medical abortion in the following countries: one in Canada, eight in the United States, one in Portugal, one in Australia, and one in the United Kingdom following mifepristone medical abortion. (3)
Conclusion
Patients should understand that safe does not mean risk free. Patients should also be informed of all potential risk before they consent to a specific medical abortion procedure and should be vigilant for symptoms after the procedure. Providers must be aware of the potential for complication and death.
Fatal infection following medical abortion has important implications both for the care of individual patients and for the international healthcare community.
Disturbing aspects of many of these medical abortion cases involved women who were young and healthy and all died remarkably rapidly after presentation of infection. (5) (30) (31) (32) (33) (34)
The global deaths in women’s reproductive healthcare are likely to be a minimum number, and the frequency of fatal infection from lethal pathogens in pregnancy remains virtually unknown. (9) (27)
The vital component to drug post-marketing surveillance in the United States and other countries is the voluntary compliance and active engagement by the health care community in recognizing and reporting adverse events.” (28)
Unfortunately, it can take years to bring these rare and important medical abortion death cases to the international community’s attention. (1) (30) (33) (35)
Highly lethal infections and the increased incidence of emerging pathogens in healthy women undergoing obstetric interventions further highlights the importance of the following: establishing global disease surveillance programs in collaboration with international agencies; improving physician awareness, management, and interventions of lethal infections; and the further study of highly virulent anerobic bacterium associated with medically induced abortions.
REFERENCES
1. Khoo, C.L., Meskhi, A. and Harris, C.P. Fatal Clostridium septicum following medical termination of pregnancy. Journal of Obstetrics and Gynaecology. July, 2013, Vols. Volume 33, No. 5, Pages 530-531.
2. MacLennan, John D. THE HISTOTOXIC CLOSTRIDIAL INFECTIONS OF MAN. NCBI. [Online] June 26, 1962. [Cited: September 19, 2013.] http://www.ncbi.nlm.nih.gov/pmc/articles/PMC441149/. PMCID: PMC441149.
3. Patterson, Monty L. Abortion Pill Risks, Just The Facts. Abortion Pill Risks. [Online] 2013. [Cited: October 8, 2013.] http://abortionpillrisks.org/.
4. Khoo, C.L. Case Study: Prophylactic Use of Antibiotics. Personal E-Mail Communications. s.l. : Monty L. Patterson of AbortionPillRisks.org, September 24, 2013.
5. Khoo, C.L. Questions_Fatal Clostridium septicum following medical termination of pregnancy. Personal E-Mail Communications. s.l. : Monty L. Patterson of AbortionPillRisks.org, August 12, 2013.
6. Patterson, Monty L. First Clostridium Septicum Death Post Medical Abortion Case Report. [Personal E-Mail Communications] Livermore : Centers for Disease Prevention & Control (CDC), September 10, 2013.
7. Patterson, Monty L. 2013_First Case of Fatal Clostridium Septicum Post Medical Abortion. [Personal E-Mail Communications] Livermore : U.S. Food and Drug Adminstration (FDA) , September 10, 2013.
8. World Health Organization. Abortion in Europe. Entre Nous 59, 2005. World Health Organization: Europe, number 59, co-published with IPPF European Network. [Online] 2005. [Cited: October 8, 2013.] http://www.euro.who.int/__data/assets/pdf_file/0004/69763/en59.pdf.
9. Creinin, Mitchell, Blumenthal, Paul and Shulman, Lee. Mifepristone-Misoprostol Medical Abortion Mortality. Medscape General Medicine, Contemporary Issues in Ob/Gyn & Women's Health, 2006;8(2):26. [Online] [Cited: September 23, 2013.] http://www.medscape.com/viewarticle/529318.
10. U.S Department of Health & Human Services. Drugs, Public Health Advisory: Sepsis and Medical Abortion, November 4, 2005 Update. FDA, U.S. Food and Drug Administration. [Online] November 04, 2005. [Cited: July 16, 2011.] http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm051734.htm.
11. U.S. Department of Health & Human Services. Drugs, Public Health Advisory: Sepsis and medical abortion with mifepristone (Mifeprex). FDA, U.S. Food and Drug Administration. [Online] March 17, 2006. [Cited: July 13, 2011.] http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm051298.htm.
12. U.S. Department of Health & Human Services. Drugs, Labeling and Regulatory History from Drugs@FDA, Mifeprex (mifepristone) Medication Guide, Rev 3: 4/22/09. FDA, U.S. Food and Drug Administration. [Online] July 19, 2011. [Cited: July 20, 2011.] http://www.fda.gov/downloads/Drugs/DrugSafety/UCM088643.pdf.
13. Mayo Clinid Staff. Medical abortion, What you can expect. Mayo Clinic. [Online] May 31, 2013. [Cited: September 24, 2013.] http://www.mayoclinic.com/health/medical-abortion/MY00819/DSECTION=what-you-can-expect.
14. Risks of mifepristone abortion in context. McGregor, JA and Equiles, O. November 2005, Contraception Journal, Volume 72, issue 5, p. 393.
15. Miech, Ralph P. Disruption of the innate immune system by mifepristone and lethal toxin of Clostridium sordellii. Journal of Organ Dysfunction. 2007, pp. 1-5, i-First article.
16. Aronoff, David M, et al., et al. Misoprostol Impairs Female Reproductive Tract Innate Immunity against Clostridium sordellii. The Journal of Immunology, vol 180, no 12, 8222-8230. [Online] June 15, 2008. [Cited: July 25, 2011.] l. http://www.jimmunol.org/content/180/12/8222.abstract.
17. Webster, Jeanette I and Sternberg, Esther M. Role of the hypothalmic-pituitary-adrenal axis, glucocorticoids and glucocorticoid receptors in toxic sequelae of exposure to bacterial and viral products. Journal of Endocrinology, 181, 207-221. [Online] May 2004. [Cited: July 25, 2011.] http://joe.endocrinology-journals.org/content/181/2/207.full.pdf.
18. Miech, Ralph P. Pathophysiology of Mifepristone-Induced Septic Shock Due to Clostridium sordellii, Annals of Pharmacotherapy, Vol 39(9). The Annals of Pharmacotherapy. [Online] September 2005. [Cited: September 12, 2011.] http://www.theannals.com/content/39/9/1483.abstract. doi: 10.1345/aph.1G189.
19. Khan, Azhar: Davenport, Kim. A reminder of the association between Clostridium septicum and colonic adenocarcinoma. International Seminars in Surgical Oncology . [Online] April 28, 2006. [Cited: September 23, 2013.] http://www.issoonline.com/content/3/1/12. doi:10.1186/1477-7800-3-12.
20. Haas, LEM, Tjan, DHT and van Zanten, ARH. Fatal Clostridium septicum infection in a young pregnant woman. The Netherlands Journal of Medicine, Letter to the Editor. July-August, 2006, Vols. 64, pages 254-5, 7.
21. Bretzke, ML, Bubrick, MP and Hitchcock, CR. Diffuse spreading Clostridium septicum infection, malignant disease and immune suppression. Surg Gynecol Obstet. 166, 1988, Vol. (3), Pages 197-9.
22. Abella, BS, et al., et al. Atraumatic Clostridial myonecrosis: case report and literature review. NCBI. [Online] [Cited: September 18, 2013.] http://www.ncbi.nlm.nih.gov/pubmed/12745042. PMID: 12745042.
23. Pelletier, JPR, et al., et al. The Role of Clostridium septicum in Paraneoplastic Sepsis. Arch Pathol Lab Med. March, 2000, Vol. 124, Pages 353-356.
24. Wiersema, B, Scheid, D and Psaradellis, T. A Rare Trifocal Presentation of Clostridium septicum Myonecrosis. ORTHOPEDICS, Volume 31, Issue 3. [Online] March 2008. [Cited: September 18, 2013.] http://www.healio.com/orthopedics/infection/journals/ortho/%7B30eec211-1970-4f5b-a738-364c876dd9d5%7D/a-rare-trifocal-presentation-of-clostridium-septicum-myonecrosis.
25. McDonald, RE and Moola, S. Clostridium septicum infection in a young pregnant patient. PubMed, US National Library of Medicine National Institutes of Health. [Online] June 5, 2012. [Cited: September 30, 2013.] http://www.ncbi.nlm.nih.gov/pubmed/22675151. doi: 10.1136/bcr-2012-006254..
26. Reeves, Matthew: Creinin, Mitchell. Mortality in Perspective: Mifepristone-Misoprostol Medical Abortion. John Hopkins advanced Studies in Medicine, OP-ED. November/December, 2006, Vol. 6, 10.
27. Berg, Cynthia J, Zane, Suzanne B and Prevention, Centers for Disease Control. Deaths from Clostridium sordellii after Medical Abortion; correspondence to the editor. The New England Journal of Medicine. [Online] April 13, 2006. [Cited: September 24, 2013.] http://www.nejm.org/doi/full/10.1056/NEJMc055539. DOI: 10.1056/NEJMc055539.
28. PBS. Frontline, Dangerous Prescription,The FDA: Hazardous to Your Health?, Interview: Paul Seligman, M.D.,M.P.H. PBS.org, WGBH educational foundation. [Online] November 13, 2003. [Cited: August 16, 2011.] http://www.pbs.org/wgbh/pages/frontline/shows/prescription/interviews/seligman.html.
29. PBS. Frontline, Dangerous prescription, The FDA: Hazardous to Your Health?, Interview: Steven Galson, M.D. PBS.org, WGBH educational foundation. [Online] November 13, 2003. [Cited: August 16, 2011.] http://www.pbs.org/wgbh/pages/frontline/shows/prescription/interviews/galson.html.
30. Fischer, M, et al., et al. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion, N Engl J Med;353:2352-60. New England Journal of Medicine. [Online] December 1, 2005. [Cited: July 20, 2011.] http://www.nejm.org/doi/pdf/10.1056/NEJMoa051620.
31. Greene, Michael F. Fatal Infections Associated with Mifepristone-Induced Abortion, N Engl J Med, 353(22):2317-8. NCBI, PubMed. [Online] December 1, 2005. [Cited: September 13, 2011.] http://www.ncbi.nlm.nih.gov/pubmed/16319378. PMID: 16319378.
32. Meites, Elissa, Zane, Suzanne and Gould, Carolyn. Fatal Clostridium sordellii Infections after Medical Abortions. The New England Journal of Medicine. [Online] September 30, 2010. [Cited: July 16, 2011.] http://www.nejm.org/doi/full/10.1056/NEJMc1001014.
33. Reis, T, et al., et al. A Clostridium sordellii fatal toxic shock syndrome post-medical-abortion in Portugal, Abstract number: R2542. ESCMID, European Society of Clinincal Microbiolgy and Infectious Diseases, Milan, Italy. [Online] May 7-10, 2011. [Cited: September 12, 2011.] http://www.eccmidabstracts.com/abstract.asp?id=93762.
34. Sinave, Christian, et al., et al. Toxic Shock Syndrome Due to Clostridium sordellii: A Dramatic Postpartum and Postabortion Disease, Volume 35, Issue 11, pp. 1441-1443. Oxford Journals, Clinical Infectious Diseases. [Online] August 7, 2002. [Cited: September 12, 2011.] http://cid.oxfordjournals.org/content/35/11/1441.full?sid=a2610f54-ca62-403f-bce5-78e2eabb4bcf. doi: 10.1086/344464.
35. Cohen, Adam, et al., et al. Toxic Shock Associated With Clostridium sordellii and Clostridium perfringens After Medical and Spontaneous Abortion. Obstetrics & Gynecology, November 2007-Volume 110 -Issue 5 -pp 1027-1033. [Online] November 2007. [Cited: July 16, 2011.] http://journals.lww.com/greenjournal/fulltext/2007/11000/toxic_shock_associated_with_clostridium_sordellii.14.aspx.
Page Last Updated: October 10, 2013

